Optipedia • SPIE Press books opened for your reference.
Absorption
Excerpt from Field Guide to Spectroscopy
There are two spectroscopically important processes: absorption and emission.
Absorption is when an atomic or molecular process takes in a photon and goes to a higher energy such that the increase in energy equals the energy of the photon:
In 1917, Albert Einstein defined a rate of stimulated absorption as
where ρ(ν) is the density of radiation having frequency ν, c is the concentration of the absorbers in the lower energy level, and B is the Einstein coefficient of stimulated absorption and is given by
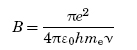
Absorption of photons is probably the most common measurement in spectroscopy. The absorptivity of a chemical substance, ε(λ), is a measure of how strongly that substance absorbs light of a particular wavelength. It is commonly expressed in molar terms and is also referred to as the molar absorptivity or the molar extinction coefficient. The absorbance, A(λ), of a sample depends on the molar absorptivity, the concentration of the chemical substance, and the distance the light passes through the sample.
D. W. Ball, Field Guide to Spectroscopy, SPIE Press, Bellingham, WA (2006).
View SPIE terms of use.

